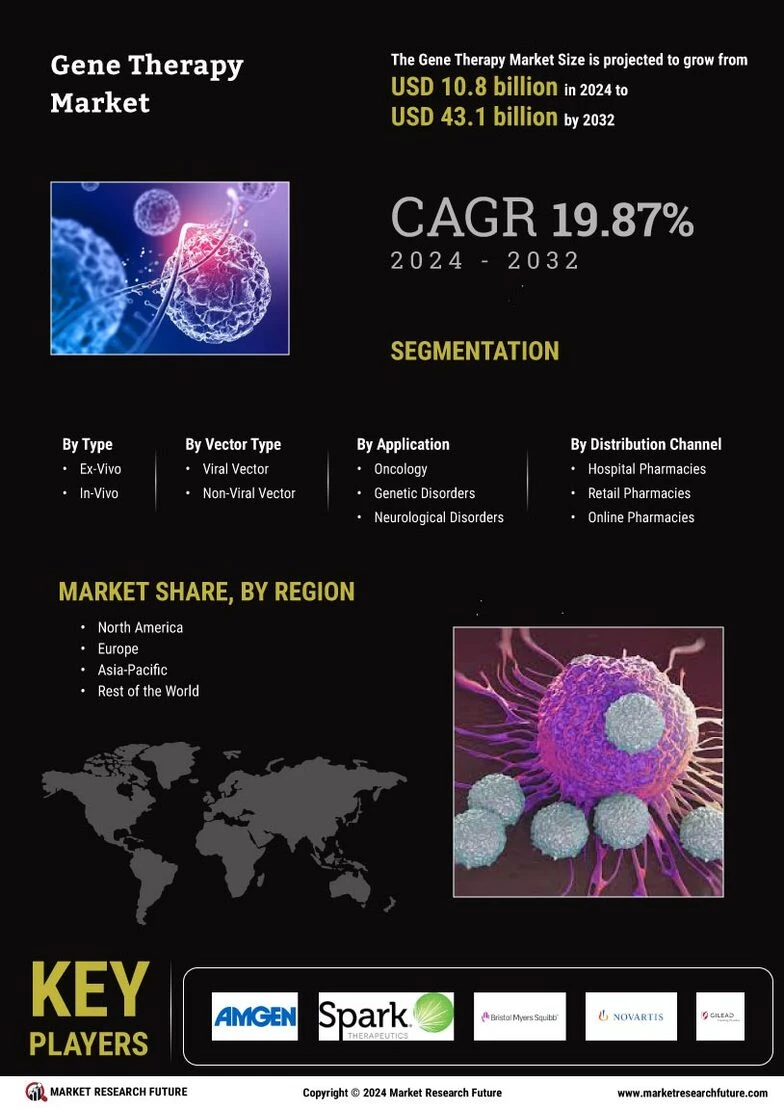
Emerging Horizons in the Gene Therapy Market: Global Landscape, Growth Dynamics, and Future Potential
The gene therapy market is rapidly transforming the global healthcare landscape by introducing advanced therapeutic solutions that target the root cause of diseases at the molecular level. Supported by expanding biotechnology research, rising clinical trial success rates, and increased investments in precision medicine, the market has experienced significant growth in recent years. The rising prevalence of genetic disorders, cancer, and rare diseases continues to fuel demand for innovative gene-modulating therapies. As a result, the global gene therapy market is witnessing accelerated revenue expansion, a growing treatment pipeline, and a sharp increase in regulatory approvals, strengthening its long-term outlook.
Market growth is further supported by the shift from traditional treatment models to targeted and personalized medicine. Companies are investing heavily in viral vectors, CRISPR-based gene editing, and non-viral delivery platforms to improve treatment safety and efficacy. A rising number of partnerships between pharmaceutical companies, academic institutes, and biotechnology firms is also shaping the competitive landscape. These collaborations are enhancing technological capabilities, increasing research productivity, and supporting large-scale manufacturing — crucial for meeting global demand. Additionally, favorable regulatory frameworks and fast-track approvals for breakthrough therapies, especially in the U.S. and Europe, are helping the gene therapy market achieve sustained momentum and wider adoption
.
Get full REports:
https://www.marketresearchfuture.com/reports/gene-therapy-market-8399
The pipeline of gene therapy products continues to expand as research efforts intensify across therapeutic categories. Oncology remains the leading segment, with multiple gene-modulating therapies in late-stage clinical trials aimed at treating melanoma, leukemia, and solid tumors. Neurological disorders such as spinal muscular atrophy (SMA), Huntington’s disease, and Parkinson’s disease are also gaining attention due to clinical advancements and increasing funding. Meanwhile, the treatment outlook for rare diseases is improving, as gene therapy offers long-term solutions for conditions that previously had no effective therapeutic options. Key pipeline expansions include innovations in adeno-associated virus (AAV) vectors, lentiviral vectors, and next-generation gene editing platforms, all of which are helping manufacturers address safety challenges and improve targeted delivery.
From a regional perspective, North America holds the largest share of the gene therapy market due to strong R&D infrastructure, high healthcare expenditure, and the presence of leading biotechnology companies. Europe follows closely, supported by advanced clinical research networks and growing regulatory support. Meanwhile, Asia-Pacific is expected to witness the fastest growth as emerging economies increase investments in biopharmaceutical innovation, expand clinical trial capabilities, and strengthen healthcare systems to support advanced therapies. This regional expansion highlights the growing global demand for curative treatment options and cutting-edge genetic technologies.
Emerging Horizons in the Gene Therapy Market: Global Landscape, Growth Dynamics, and Future Potential
The gene therapy market is rapidly transforming the global healthcare landscape by introducing advanced therapeutic solutions that target the root cause of diseases at the molecular level. Supported by expanding biotechnology research, rising clinical trial success rates, and increased investments in precision medicine, the market has experienced significant growth in recent years. The rising prevalence of genetic disorders, cancer, and rare diseases continues to fuel demand for innovative gene-modulating therapies. As a result, the global gene therapy market is witnessing accelerated revenue expansion, a growing treatment pipeline, and a sharp increase in regulatory approvals, strengthening its long-term outlook.
Market growth is further supported by the shift from traditional treatment models to targeted and personalized medicine. Companies are investing heavily in viral vectors, CRISPR-based gene editing, and non-viral delivery platforms to improve treatment safety and efficacy. A rising number of partnerships between pharmaceutical companies, academic institutes, and biotechnology firms is also shaping the competitive landscape. These collaborations are enhancing technological capabilities, increasing research productivity, and supporting large-scale manufacturing — crucial for meeting global demand. Additionally, favorable regulatory frameworks and fast-track approvals for breakthrough therapies, especially in the U.S. and Europe, are helping the gene therapy market achieve sustained momentum and wider adoption
.
Get full REports:https://www.marketresearchfuture.com/reports/gene-therapy-market-8399
The pipeline of gene therapy products continues to expand as research efforts intensify across therapeutic categories. Oncology remains the leading segment, with multiple gene-modulating therapies in late-stage clinical trials aimed at treating melanoma, leukemia, and solid tumors. Neurological disorders such as spinal muscular atrophy (SMA), Huntington’s disease, and Parkinson’s disease are also gaining attention due to clinical advancements and increasing funding. Meanwhile, the treatment outlook for rare diseases is improving, as gene therapy offers long-term solutions for conditions that previously had no effective therapeutic options. Key pipeline expansions include innovations in adeno-associated virus (AAV) vectors, lentiviral vectors, and next-generation gene editing platforms, all of which are helping manufacturers address safety challenges and improve targeted delivery.
From a regional perspective, North America holds the largest share of the gene therapy market due to strong R&D infrastructure, high healthcare expenditure, and the presence of leading biotechnology companies. Europe follows closely, supported by advanced clinical research networks and growing regulatory support. Meanwhile, Asia-Pacific is expected to witness the fastest growth as emerging economies increase investments in biopharmaceutical innovation, expand clinical trial capabilities, and strengthen healthcare systems to support advanced therapies. This regional expansion highlights the growing global demand for curative treatment options and cutting-edge genetic technologies.